T-cell activation requires the inflow of extracellular calcium, though mechanistic particulars relating to such activation are usually not totally outlined. Here, we present that P2X(7) receptors play a key position in calcium inflow and downstream signaling occasions related to the activation of T cells.
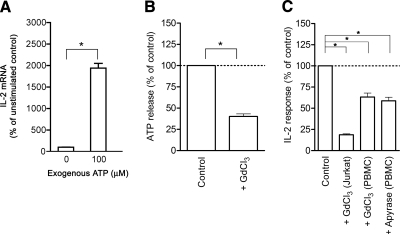
By real-time PCR and immunohistochemistry, we discover that Jurkat T cells and human CD4(+) T cells categorical considerable P2X(7) receptors. We present, utilizing a novel fluorescent microscopy approach, that T-cell receptor (TCR) stimulation triggers the fast release of ATP (<100 microM).
This release of ATP is required for TCR-mediated calcium inflow, NFAT activation, and interleukin-2 (IL-2) manufacturing. TCR activation up-regulates P2X(7) receptor gene expression.
Removal of extracellular ATP by apyrase or alkaline phosphatase therapy, inhibition of ATP release with the maxi-anion channel blocker gadolinium chloride, or <em>siRNA</em> silencing of P2X(7) receptors blocks calcium entry and inhibits T-cell activation.
Moreover, lymphocyte activation is impaired in C57BL/6 mice that categorical poorly useful P2X(7) receptors, in comparison with <em>management</em> BALB/c mice, which categorical totally useful P2X(7) receptors.
We conclude that ATP release and autocrine, <em>optimistic</em> suggestions by way of P2X(7) receptors is required for the efficient activation of T cells.
IL-27 inhibits the event of regulatory T cells through STAT3.
Regulatory CD4+ T cells are vital for the homeostasis of the immune system and their absence correlates with autoimmune issues.
Here, we examine the capability of IL-27, a cytokine with pro- and anti-inflammatory properties, to manage the era of reworking development issue beta (TGFbeta)-inducible forkhead field P3 (Foxp3)-optimistic regulatory T (Treg) cells. Our outcomes exhibit that IL-27 inhibits the acquisition of the Treg phenotype on the stage of Foxp3, CD25 and CTLA-4 (CD152) expression in addition to the suppressive perform.
In distinction to TGFbeta-induced Treg cells, the cells generated after differentiation within the presence of TGFbeta and IL-27 maintained the flexibility for IL-2 and tumour necrosis issue alpha (TNFalpha) manufacturing.
The inhibitory impact of IL-27 on Treg era was a minimum of partially sign transducer and activator of transcription 3 (STAT3) dependent as examined by focused STAT3 protein inhibition utilizing small interfering RNA (siRNA), whereas STAT1-dependent indicators appeared to oppose the STAT3 indicators.
In flip, TGFbeta blocked IL-27-induced T(h)1 differentiation. Thus, IL-27 and TGFbeta mutually management their results on CD4+ T-cell differentiation, whereby IL-27 favours inflammatory circumstances by way of a STAT3-dependent inhibition of Treg era.
