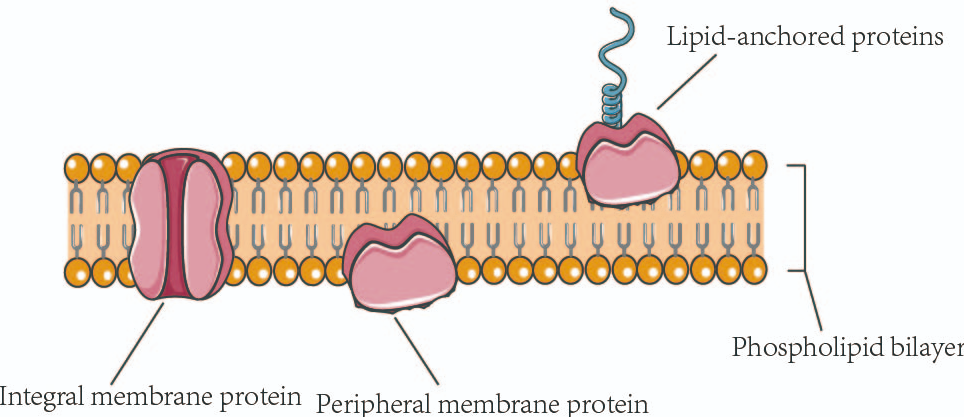
The proteins of membrane , embedded in the phospholipid bilayer of the cell, play a critical role in the maintenance of many cellular functions such as signal transduction, maintaining cellular integrity, intra transport and extracellular and intercellular communication between others.
One of the requirements for carrying out structural studies of membrane proteins is the achievement of an abundant source of it, either a natural source or heterologous overexpression using molecular biology techniques. Although some membrane proteins are very abundant in certain tissues, those of greatest interest, involved in intercellular communication and in the regulation of transmembrane concentrations of ions and metabolites, are normally present at very low levels, so that heterologous expression in these cases is a fundamental procedure, and in many cases this is not an easy task.
On the other hand, obtaining antibodies that recognize conformational epitopes of membrane proteins is an invaluable tool for its ability to bind to critical receptor regions for the performance of a certain function, as well as to detect proteins located in the cell surface. The development of antibodies against membrane proteins such as GPCRs, is extremely complicated using traditional strategies and protocols.
In this post we will try to give you some tips to optimize the expression and purification of membrane proteins in heterologous systems, as well as the production of antibodies against them.
Challenges In The Recombinant Expression Of Membrane Proteins
Recombinant protein expression basically includes four steps: vector construction, expression, purification, and characterization. For the specific case of membrane proteins , the different expression systems offer the advantages and disadvantages detailed below:
Due to the requirements of membrane proteins to associate with cell membranes, their expression in a heterologous system can become a great challenge. Often, recombinant expression of membrane proteins can result in the formation of aggregates or misfolding of the protein.
The use of living cell systems or preparations of cell-derived membranes can help improve the stability of membrane proteins, although the application of this technique has its limitations: very low concentration of target protein, heterogeneity of receptors and contaminants lipids, which can result in low sensitivity and high experimental variability.
The limited expression on the surface may be due to several factors: some membrane proteins are transcribed and translated at very low levels due to incompatibility between promoters, cells or growth conditions. Other proteins may have intrinsic limitations in terms of folding or assembling the different subunits, and the attempt of overexpression may exceed the cellular capacity resulting in premature degradation.
It should also be borne in mind that over-expressed membrane proteins can be toxic to cells due to their biological function, their activity or their activation by serum components, and this can vary depending on cell type and growth conditions.
Tips To Optimize The Membrane Protein Expression Process:
- Overexpression is the main bottleneck during the production process of recombinant membrane proteins. It is often useful to test the expression in parallel on different hosts and / or strains to increase the probability of success.
- In E. coli, the levels of membrane protein expression, even in the best cases, are usually well below the usual expression levels for soluble proteins.
- A moderate growth and expression range can be beneficial in purifying this type of protein to avoid the formation of inclusion bodies in E. coli. To achieve this, the use of weak promoters, low inducer concentration and the reduction of the growth temperature after induction can be resorted to.
Tips To Optimize The Membrane Protein Purification Process:
- Know in detail what is described in the literature on the properties of the target protein.
- Carry out small-scale extraction and solubilization experiments, in order to optimize detergents, salt concentration, pH, protease inhibitors, additives …
- Minimize isolation time.
- Dissolve the protein in the minimum detergent / protein ratio.
- Avoid intensive slipping, which could affect activity without affecting the structure.
- Assess a first enrichment step without using detergents.
- Membrane proteins tend to be less soluble at high ionic strengths, so it is always recommended that the NaCl concentration be relatively low.
- Membrane proteins often form aggregates. It is recommended to control the factors that may induce or favor this aggregation, such as the detergent ratio, the pH …

Challenges In The Production Of Antibodies Against Membrane Proteins
As we said before, antibodies that recognize conformational epitopes of membrane proteins are very valuable tools for their ability to bind to receptor regions critical to the performance of a certain function, and to detect proteins located on the cell surface.
But obtaining this type of antibody is a difficult and complex process, mainly due to three factors:
- Most epitopes require membranes to configure their structure and purification. Strategies to remove such membranes often denature proteins by destroying epitopes.
- Epitopes are made up of short and discontinuous sequences, which, being unable to fold or assemble together, represent an obstacle if the traditional technology of recombinant peptides or proteins is to be used.
- The performance in the expression of membrane proteins is usually very low, so obtaining enough material for immunization is often difficult.
Faced with these difficulties, there are novel strategies that try to overcome these problems. Among the most important are:
- DNA immunization technology (Allows antigen production to take place in vivo, avoiding the need to express and purify the membrane protein in vitro.)
- Alternative options for the design of antigens (DNA, extracellular domain, peptides, cells …)
- Patented antigenic modification technologies (Designed to break immune tolerance and produce antibodies against proteins with high homology such as GPCRs)
- Optimized high performance screening (directly selecting the antibodies that recognize the antigen on the cell surface in its native conformation using capture ELISA, FACS …)