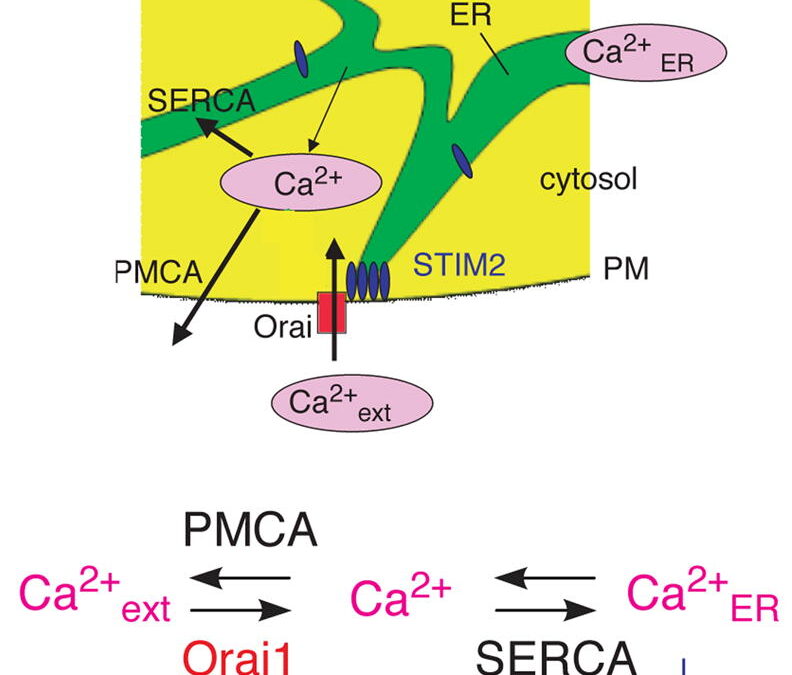
Deviations in basal Ca2+ ranges intrude with receptor-mediated Ca2+ signaling in addition to endoplasmic reticulum (ER) and mitochondrial perform.
While faulty basal Ca2+ regulation has been linked to numerous ailments, the regulatory mechanism that controls basal Ca2+ is poorly understood.
Here we carried out an siRNA display of the human signaling proteome to establish regulators of basal Ca2+ focus and discovered STIM2 because the strongest optimistic regulator.
In distinction to STIM1, a lately found sign transducer that triggers Ca2+ inflow in response to receptor-mediated depletion of ER Ca2+ shops, STIM2 activated Ca2+ inflow upon smaller decreases in ER Ca2+. STIM2, like STIM1, brought on Ca2+ inflow through activation of the plasma membrane Ca2+ channel Orai1.
Our research locations STIM2 on the heart of a feedback module that retains basal cytosolic and ER Ca2+ concentrations inside tight limits.
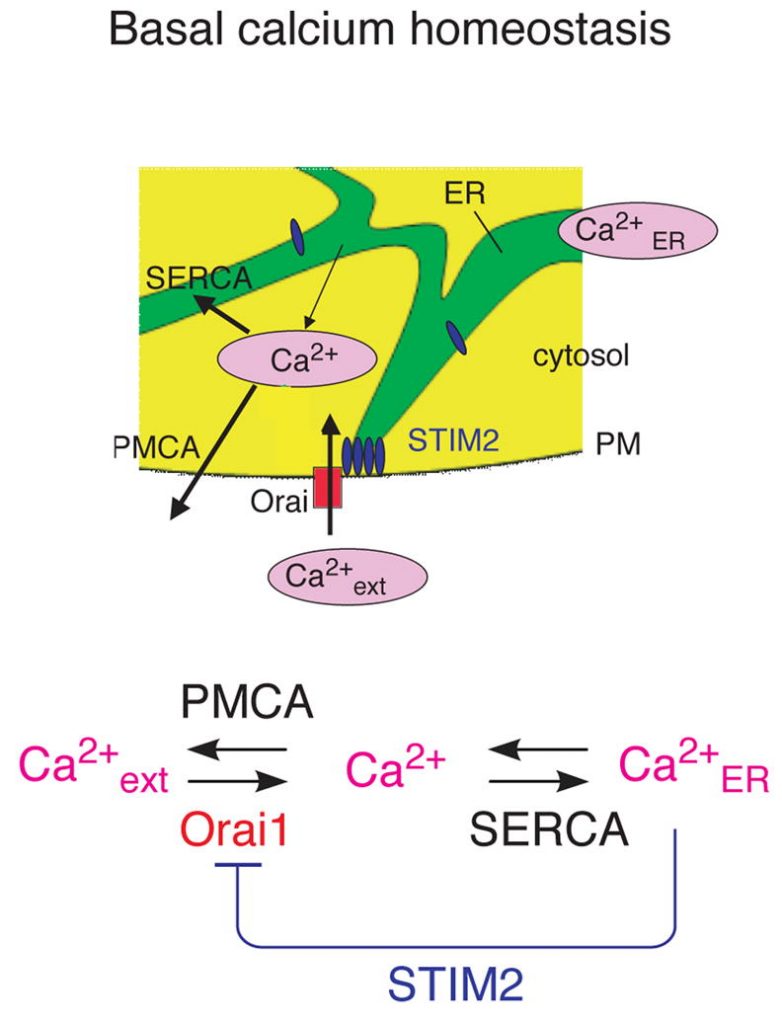
STIM2 is a feedback regulator that stabilizes basal cytosolic and endoplasmic reticulum Ca2+ ranges.
C9ORF72, implicated in amytrophic lateral sclerosis and frontotemporal dementia, regulates endosomal trafficking.
Intronic growth of a hexanucleotide GGGGCC repeat within the chromosome 9 open studying body 72 (C9ORF72) gene is the main reason behind familial amyotrophic lateral sclerosis (ALS) and frontotemporal dementia.
However, the mobile perform of the C9ORF72 protein stays unknown. Here, we reveal that C9ORF72 regulates endosomal trafficking.
C9ORF72 colocalized with Rab proteins implicated in autophagy and endocytic transport: Rab1, Rab5, Rab7 and Rab11 in neuronal cell traces, main cortical neurons and human spinal twine motor neurons, in line with earlier predictions that C9ORF72 bears Rab guanine trade issue exercise.
Consistent with this notion, C9ORF72 was current within the extracellular house and as cytoplasmic vesicles.
Depletion of C9ORF72 utilizing siRNA inhibited transport of Shiga toxin from the plasma membrane to Golgi equipment, internalization of TrkB receptor and altered the ratio of autophagosome marker mild chain 3 (LC3) II:LC3I, indicating that C9ORF72 regulates endocytosis and autophagy.
C9ORF72 additionally colocalized with ubiquilin-2 and LC3-optimistic vesicles, and co-migrated with lysosome-stained vesicles in neuronal cell traces, offering additional proof that C9ORF72 regulates autophagy.
Investigation of proteins interacting with C9ORF72 utilizing mass spectrometry recognized different proteins implicated in ALS; ubiquilin-2 and heterogeneous nuclear ribonucleoproteins, hnRNPA2/B1 and hnRNPA1, and actin. Treatment of cells overexpressing C9ORF72 with proteasome inhibitors induced the formation of stress granules optimistic for hnRNPA1 and hnRNPA2/B1.
Immunohistochemistry of C9ORF72 ALS affected person motor neurons revealed elevated colocalization between C9ORF72 and Rab7 and Rab11 in contrast with controls, suggesting potential dysregulation of trafficking in sufferers bearing the C9ORF72 repeat growth. Hence, this research identifies a position for C9ORF72 in Rab-mediated mobile trafficking.